
threatened

Illustration from Sudworth (1908).

Photo taken at Point Lobos State Reserve, Monterey County by Charles Webber © California Academy of Sciences.

Photo taken on the Cal Poly Swanton Pacific Ranch, Santa Cruz County © 2006 Dylan Neubauer.

Photo taken at the Presidio, Monterey County © Dean W. Taylor.
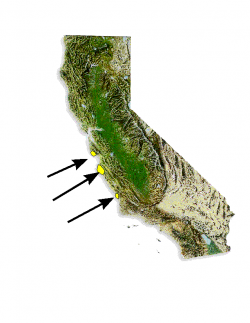
Distribution of Pinus radiata.



This fact sheet was prepared by Grey F. Hayes, Dean W. Taylor, and Dylan M. Neubauer under award NA04N0S4200074 from the National Oceanic and Atmospheric Administration (NOAA), U.S. Department of Commerce (DOC). The statements, findings, conclusions, and recommendations are those of the authors and do not necessarily reflect the views of the NOAA or the DOC.
© Copyright 2006, Elkhorn Slough Coastal Training Program
Last updated: Sep 5, 2007 12:12
Common Names - Monterey pine
Family - Pinaceae (Pine Family)
State Status - none
Federal Status - none
Habitat
Hills and gentle to moderate slopes within the maritime summer-fog zone in closed-cone pine forest—or co-occurring with a variety of tree species including pine, cypress, redwood, Douglas-fir, and oak. Soils are derived from a variety of parent materials, though most often marine sediments and are acidic to extremely acidic thus nutritionally poor. Often a clay layer is present, and a thick layer of duff is common in higher quality sites (McDonald and Laacke). Mainland populations generally occur at < 500 m.
Key Characteristics
Large trees to 38 m tall, generally unbranched (Monterey stand) or highly branched (Año Nuevo stand); mature bark black, deeply grooved; mature crown irregular, round-topped; needles in fascicles of 3 (2 on the island form), 6–15 cm long, dark green; female cones recurved, pendant, 6–15 cm long, asymmetric, light brown, opening slowly 2nd year, persistent < 25 years; proximal scale tip knobs < 2 cm, rounded, minute-prickled; seed < wing (Haller and Vivrette 2013).
Flowering Period
Male cones March to May; female cones present throughout the year
Reference Populations
Año Nuevo State Park (San Mateo County); Big Basin Redwoods State Park (Santa Cruz County); Point Lobos State Reserve, Del Monte Forest (Monterey County).
Global Distribution
Three discrete native populations exist in central coastal California (Santa Cruz/San Mateo, Monterey, and San Luis Obispo counties), and two off the west coast of Baja California, Mexico, on Isla Cedros and Isla Guadalupe. More than 10 million acres are planted for timber harvest worldwide—especially in Australia, New Zealand, Chile, South Africa, Spain, and the British Isles.
Taxonomy and Nomenclature
In her discussion of the taxonomic history of Monterey pine, Millar (1986) observes that “[t]he 19th-century taxonomy of Monterey pine was complex due not only to poor communication among pine botanists and hence redundant naming, but also to the prevailing typological species concepts, whereby even slight phenotypic variants (especially cone) were considered separate species.”
The first name assigned to this species was Pinus californiana Loise., and was based on plants grown from seed collected at Monterey in 1786 by Collignon, the gardener for the French La Pérouse expedition. Because of confusion in the labeling of plant material, the section of the description dealing with seeds clearly referred to a different species of pine (Lemmon 1893). Therefore, this name was “largely ignored” by other botanists, who considered “it inadequately described and the tree poorly identified” (Millar 1986). A variety of other names ensued, including the 1837 P. radiata D. Don, which was described on the basis of “a few cones” from material collected by Thomas Coulter, though Don “rightly conjectured” the needles “to be in threes” (Lemmon 1894; Millar 1986). The following year, Loudon described P. insignis—a name originally assigned by Douglas—based on a more complete evaluation of habit, foliage, fruit, and seed morphology, from collections by Douglas (Lemmon 1893, Millar 1986). By the 1880s, the name P. insignis was “adopted by most botanists of the period” (Lemmon 1893) and was applied to “Monterey pine populations at Año Nuevo and Cambria” (Millar 1986). Despite the fact that this was “the first exact description,” Lemmon (1893) emphasized that the name P. radiata had “priority by seven years,” and he held that this name was the name that “must hold for the Monterey pine.” He was primarily responsible for promoting the usage of P. radiata over that of P. insignis for “mainland Californian populations,” and in 1895 he “officially discarded” the latter, whereupon, subsequently, the former gained widespread usage (Millar 1986).
Trees from the Cambria grove have been treated as a distinct variety, but later evidence did not support this separation. Natural hybrids have been observed in the Ano Nuevo population between P. radiata and P. attenuata. (Artificial crosses have been treated as P. x attenuradiata Stockwell & Righter; 1946.)
The identity of the two island populations has been the subject of ongoing debate. They have been allied with P. muricata and P. radiata. Genetic studies in the 1960s and later confirmed the relationship of the Guadalupe Island pine to P. radiata, and it has been commonly referred to as var. binata (Engelm.) Lemmon. The Cedros island pine wasn’t legitimately published as P. radiata [var. cedrosensis (J. T. Howell) Silba] until 1990, though described in 1980 by Axelrod. Current treatments (Kral 1993; Haller and Vivrette 2013) do not recognize these taxa.
Biology, Ecology, and Evolutionary History
Monterey pine is a fast-growing, relatively short-lived closed-cone pine. Closed-cone pines generally are reliant on high-intensity fire to open the cones and release the stored seedbank, though in the case of Monterey pine, cones open and close intermittently releasing some seeds, depending on humidity and temperature. Its shade-tolerance is intermediate, and maximum seed production does not occur until the tree is well into its first or second decade of life (McDonald and Laacke n.d.). The optimal seedbed is bare mineral soil such as that created by fire (McDonald and Laacke n.d.; Millar 1998) in full sun or light shade.
Monterey pine shares habitat with other closely related closed-cone pines—Bishop pine (P. muricata) in Monterey and Cambria, and knobcone pine (P. attenuata) in the Año Nuevo area—and hybrids with the latter species have been observed there. In the opinion of botanist James A. West, the Año Nuevo/Swanton stand of Monterey pine may be an ancient hybrid swarm formed with knobcone pine but now reproductively isolated from it (West 2015).
Based on fossil, biogeographic, and climatological evidence, it has been hypothesized that P. radiata was widely distributed along the California coast over the past two million years in disjunct locations, “expanding and contracting in response to shifts in climate and … fire-regimes,” and favoring intermediate conditions (Millar 1998, 1999). Its evolutionary history has adapted the species to “small population sizes, to fluctuations in size, to colonizations of new locations, and even to local extirpations” (Millar 1998). Preliminary genetic evidence points to a “strong level of population differentiation,” though not all populations have been tested, while “genetic patterns within populations” have not been well studied (Rogers 2002).
Monterey pine forests support a number of sensitive species including Monterey cypress (Hesperocyparis macrocarpa), Hooker’s manzanita (Arctostaphylos hookeri subsp. hookeri), sandmat manzanita (Arctostaphylos pumila), Pacific Grove clover (Trifolium polyodon), Monterey clover (Trifolium trichocalyx), Hickman’s cinquefoil (Potentilla hickmanii), Hickman’s onion (Allium hickmanii), and Yadon’s rein orchid (Piperia yadonii) [see Factsheets on these species].
Threats
Habitat loss/fragmentation due to development remains the greatest threat to the remaining portions of the native populations of this charismatic, iconic species, the majority of which occur on private lands. These populations are invaluable as they contain the genetic diversity (and the future) of this widely planted species.
When the understory is cleared, the health of the pine forest as a whole suffers though individual pine trees may persist for many years. Roads are problematic as they hinder range expansion and inhibit the flow of genes, possibly resulting in inbreeding depression. Genetic contamination to indigenous stands by planted, non-local Monterey pines is also a concern.
The reduction in natural fire frequency resulting from development has interfered with reproductive cycles and encouraged the spread of pests and pathogens. These include bark beetles and pitch canker (Fusarium circinatum), a non-native fungus first observed in California in Santa Cruz County in 1986 and now occurring in 18 coastal counties, with a higher prevalence in the fog zone and in fragmented forest (UCANR 2013). Though pitch canker has contributed to increased mortality among Monterey pine, the threat is not as great as initially anticipated. Certain trees may develop resistance to the pest over time, or have genetic resistance—though this may only hold true for the short term as new strains appear (UCANR 2013). Habitat preservation to encourage regeneration of resistant strains has been recommended as the most ecologically sound mitigation measure.
Another potential long-term threat is climate change—any decrease or change in the distribution of fog could reduce the amount of moisture the trees receive from fog drip.
Conservation
In 1999, the California Native Plant Society (CNPS) applied to have the Monterey pine listed by the state, but the petition application was withdrawn as California Department of Fish and Wildlife was not able to process the petition within the allotted time period (Nedeff, pers. comm.). CNPS recommends that preservation efforts should be afforded to larger stands (> 20 acres), fire-resistant construction should be required, and replanting should be from site-specific seed stock. For the Monterey stand, CNPS encourages the preservation of forest in various elevational zones on the Peninsula, the maintenance of connectivity between patches and other sensitive areas, and stresses the importance of preserving the entire forest community rather than focusing on the significance individual trees.
Along with preservation of currently existing stands of Monterey pine, other strategies have been put forth. Since the historic range has naturally expanded and contracted, preservation of—and even carefully thought out—intentional planting in currently unused habitat (historic and otherwise) may be what is required since development now hinders natural expansion (Millar 1998).
Rogers (2002) stresses that the most “critical conservation action” is the purchase of land in order “to protect remaining forests from degradation or conversion to other uses.” She highlights the effectiveness of conservation easements on private lands and the educational and monitoring activities of NGOs. Additional recommendations include the establishment of in situ “genetic reserves” for each population and the conservation of “outliers,” which may harbor genetic diversity useful in a changing environment (Rogers 2002, 2004).
References
Axelrod, D. I. 1980. History of the maritime closed-cone pines, Alta and Baja California. University of California Publications in Geological Sciences, Vol. 120.
Axelrod, D. I. and F. Govean. 1996. An early Pleistocene closed-cone pine forest at Costa Mesa, Southern California. International Journal of Plant Sciences 157(3):323–329.
Bannister, M. H. 1958. Evidence of hybridization between Pinus attenuata and P. radiata in New Zealand. Transactions of the Royal Society of New Zealand 85(2):217–225.
California Native Plant Society (CNPS), Rare Plant Program. 2010. Pinus radiata, in Inventory of Rare and Endangered Plants (online edition, v8-02). California Native Plant Society, Sacramento, CA. http://www.rareplants.cnps.org/detail/1378.html [accessed 21 February 2015].
Critchfield, W. B. 1967. Crossability and relationships of the closed-cone pines. Silvae Genetica 16:89–97.
Griffin, J. R. and W. B. Critchfield. 1976. The distribution of forest trees in California. Research paper PSW-82. USDA, Forest Service, Pacific Southwest Research Station, Berkeley, CA.
Haller, J. R. and N. J. Vivrette. 2013. Pinus radiata, in Jepson Flora Project (eds.). Jepson eFlora, http://ucjeps.berkeley.edu/cgi-bin/get_IJM.pl?tid=38300 [accessed 21 February 2015].
Hardy, A. D. 1928. Pinus insignis: its romantic history. The Argus (Melbourne, Victoria), p. 6. http://trove.nla.gov.au/ndp/del/article/3962629 [accessed 4 January 2016].
Kral, R. 1993. Pinus. In: Flora of North America Editorial Committee, eds. 1993+. Flora of North America North of Mexico. 19+ vols. New York and Oxford. Vol. 2. http://www.efloras.org/florataxon.aspx?flora_id=1&taxon_id=233500951 [accessed 5 January 2016].
Lemmon, J. G. 1893. Notes on West American coniferae—III, bibliography of two Californian pines. Erythea 1:224–231.
McDonald, P. M. and R. J. Laacke. No date. USDA Forest Service, Northeastern Area. Pinus radiata D. Don Monterey Pine. http://www.na.fs.fed.us/spfo/pubs/silvics_manual/Volume_1/pinus/radiata.htm [accessed 6 January 2015].
Millar, C. I. 1986. The California closed-cone pines (Subsection Oocarpae Little & Critchfield): a taxonomic history and review. Taxon 35(4):657–670.
Millar, C. I. 1998. Reconsidering the conservation of Monterey pine. Fremontia 26 (3):12–16.
Millar, C. I. 1999. Evolution and biogeography of Pinus radiata, with a proposed revision of its Quaternary history. New Zealand Journal of Forestry Science 29(3):335–365.
Moran, G. F., J. C. Bell, and K. G. Eldridge. 1988. The genetic structure and the conservation of the five natural-populations of Pinus radiata. Canadian Journal of Forest Research 18(5):506–514.
Nedeff. N. Personal communication [1 June 2015].
Perry, F. 2004. The Monterey pine through geologic time. Monterey Bay Paleontological Society Bulletin, July-September. Available at Understanding Evolution. http://evolution.berkeley.edu/evolibrary/article/montereypines_01 [accessed 4 January 2016].
Rogers, D. L. 2002. In situ genetic conservation of Monterey Pine (Pinus radiata D. Don): information and recommendations. Genetic Resource Conservation Program, Report no. 26. University of California, Davis, CA.
Rogers, D. L. 2004. In situ genetic conservation of a naturally restricted and commercially widespread species, Pinus radiata. Forest Ecology and Management 197:311–322.
Silba, J. 1990. Supplement to Coniferae, census II. Phytologia 68(1):60–61.
Stephens, S. L., D. C. Pirto, and D. F. Caramagno. 2004. Fire regimes and resultant forest structure in the Native Año Nuevo Monterey pine (Pinus radiata) forest, California. American Midland Naturalist 152:25–36. http://digitalcommons.calpoly.edu/cgi/viewcontent.cgi?article=1010&context=nrm_fac [accessed 5 January 2016].
Stockwell, P. and F. I. Righter. 1946. The fertile species hybrid between knobcone and Monterey pines. Madroño 8(5):157–160.
Storer, A. J., P. Bonello, T. R. Gordon, and D. L. Wood. 1999. Evidence of resistance to the pitch canker pathogen (Fusarium circinatum) in native stands of Monterey pine (Pinus radiata). Forest Science 45:500–505.
Sudworth, G. B. 1908. Forest Trees of the Pacific Slope. United States Government Printing Office, Washington, D.C.
University of California Agriculture & Natural Resources (UCANR). 2013. Pests in gardens and landscapes: pitch canker. http://www.ipm.ucdavis.edu/PMG/PESTNOTES/pn74107.html [accessed 7 January 2016].
Valkonen, S. and D. D. Piirto. 2005. Structure and development of pitch canker infected Monterey pine stands at Año Nuevo, California. Forest Ecology and Management 213:160–174.
Vogl, C., A. Karhu, G. Moran, and O. Savolainen. 2002. High resolution analysis of mating systems: inbreeding in natural populations of Pinus radiata. Journal of Evolutionary Biology 15(3):433–439.
West, J. A. 2015. Traversing Swanton Road. http://arboretum.ucsc.edu/pdfs/traversing-swanton.pdf [accessed 6 January 2016].
Reviewers
Paul Cylinder, Corky Matthews, Deborah Rogers, and James A. West (August 2007); Nicki Nedeff (June 2015).
Conservation and Threats
In 1999, the California Native Plant Society (CNPS) applied to have the Monterey pine listed by the state, but the petition application was withdrawn as California Department of Fish and Wildlife was not able to process the petition within the allotted time period (Nedeff, pers. comm.). CNPS recommends that preservation efforts should be afforded to larger stands (> 20 acres), fire-resistant construction should be required, and replanting should be from site-specific seed stock. For the Monterey stand, CNPS encourages the preservation of forest in various elevational zones on the Peninsula, the maintenance of connectivity between patches and other sensitive areas, and stresses the importance of preserving the entire forest community rather than focusing on the significance individual trees.
Along with preservation of currently existing stands of Monterey pine, other strategies have been put forth. Since the historic range has naturally expanded and contracted, preservation of—and even carefully thought out—intentional planting in currently unused habitat (historic and otherwise) may be what is required since development now hinders natural expansion (Millar 1998).
Rogers (2002) stresses that the most “critical conservation action” is the purchase of land in order “to protect remaining forests from degradation or conversion to other uses.” She highlights the effectiveness of conservation easements on private lands and the educational and monitoring activities of NGOs. Additional recommendations include the establishment of in situ “genetic reserves” for each population and the conservation of “outliers,” which may harbor genetic diversity useful in a changing environment (Rogers 2002; 2004).
